Asymmetric synthesis, crucial in organic chemistry for producing single enantiomers, relies on techniques like biocatalysis, utilizing enzymes to ensure high selectivity. Halohydrin dehalogenases (HHDHs) have gained attention for their biocatalytic prowess, opening up various synthetic possibilities. Despite limitations like low substrate solubility, low substrate concentration, and possible enzyme inhibitions, HHDHs offer promise for diverse chemoenzymatic syntheses.
Scheme 1. Preliminary results, R. J. Kolman, P. Švaco, M. Majerić Elenkov, I. Dokli, Advanced Synthesis and Catalysis, 366 (2024) 5066-5072; DOI: 10.1002/adsc.202400734.
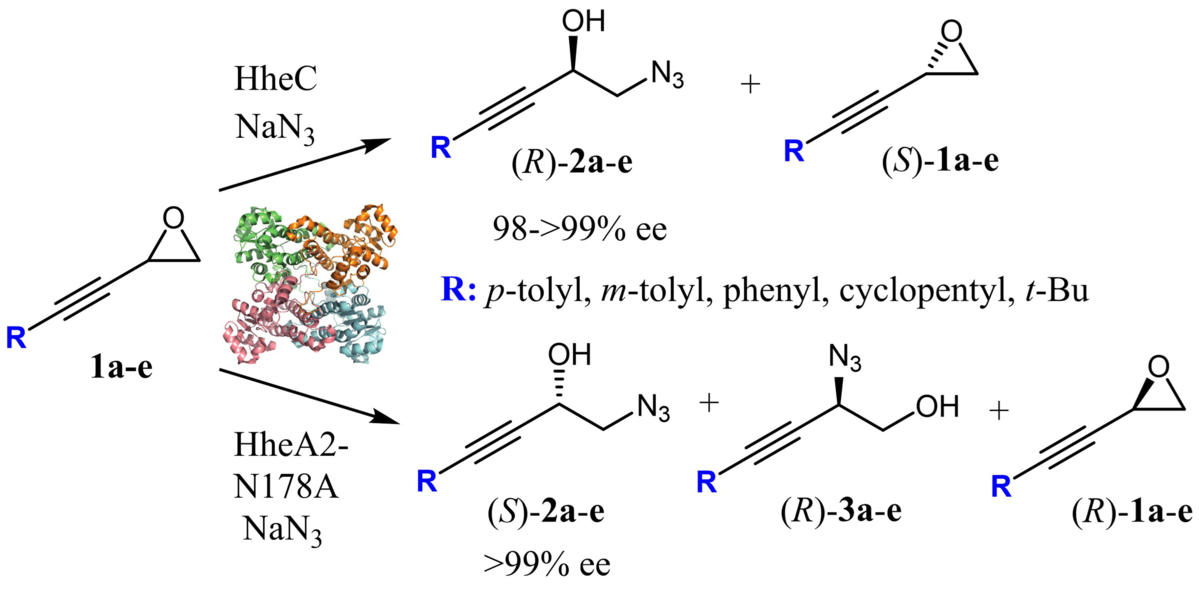
Within this project we aim to expand the substrate scope of HHDHs to propargylic and homopropargylic epoxides aiming to obtain valuable chiral alcohols with versatile reactivity. Propargylic and homopropargylic epoxides and alcohols, can undergo various inter- and intramolecular reactions due to the presence of the triple bond. These reactions can lead to the formation of diverse heterocyclic compounds, such as furans, pyrroles, triazoles, and allenic compounds. Such versatility makes propargylic compounds highly desirable as intermediates in the synthesis of organic compounds, including biologically active substances, natural products, and pharmaceuticals. Despite their significance, the stereoselective preparation of propargylic and homopropargylic epoxides and alcohols remains a challenge in organic synthesis. By exploring enzymatic resolution as an environmentally friendly and accessible alternative method, this project aims to unlock the synthetic potential of propargylic compounds, paving the way for the efficient production of valuable chiral building blocks.
Team members
Maja Majerić Elenkov
Anamarija Knežević
Petra Švaco
Danijel Glavač
Conferences
- Organic chemistry in Croatia, Croatian Academy of Sciences and Arts, Zagreb, June 9, 2025
Irena Dokli, Synthesis and Halohydrin-dehalogenase Catalyzed Kinetic Resolution of Propargylic Epoxides; invited lecture.
- 23rd European Symposium on Organic Chemistry in Copenhagen, June 29 - July 3, 2025
Danijel Glavač, Robert J. Kolman, Maja Majerić Elenkov, Irena Dokli, Biocatalytic Access to Chiral Propargylic Compounds via HHDH-Mediated Epoxide Resolution; poster presentation.
Funding: